Two aloof elements would bind under pressure, perhaps forming a superconductor, researchers say
It is the woeful truth: lithium (Li) and beryllium (Be) -- elements three and four, respectively, on the periodic table -- do not like each other.
They are the two lightest known metals in the universe and have plenty in common. Regrettably, that includes a distaste for contact with each other.
But that may soon change, say Cornell researchers. Given some healthy encouragement, the scientists have found that the two elements could abandon their mutual antipathy for something closer to, well, neighborly rapprochement. And they could do so in some surprisingly complex, and potentially very useful, ways.
The research, supported by the National Science Foundation, appears in the Jan. 24 issue of the journal Nature.
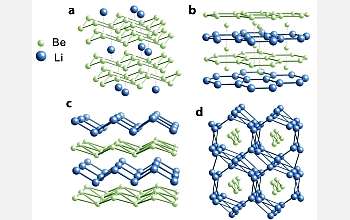
Using computer modeling complemented by what co-author Roald Hoffmann, the 1981 chemistry Nobel laureate and Cornell's Frank H.T. Rhodes Professor in Humane Letters Emeritus, calls "chemical intuition," the scientists have discovered hypothetical conditions in which Li and Be, squeezed together under hundreds of thousands of atmospheres of pressure, bind to form stable -- and possibly superconducting -- alloys.
While the elements are two of the simplest around, their combination would be highly complex, with layers of electrons forming quasi two-dimensional sheets between tightly packed nuclei. "It's nothing you would have expected," said Hoffmann.
Ji Feng, Ph.D. '07, now a postdoctoral researcher at Harvard, is lead author of the Nature paper, with co-authors Richard Hennig, a Cornell assistant professor in materials science and engineering, and Neil W. Ashcroft, the Horace White Professor of Physics Emeritus.
Ashcroft's research group first predicted in the 1990s that Li alone at high pressure could be a superconductor. That prediction was quickly confirmed. Beryllium on its own is not a superconductor, said Ashcroft, but many of the element's characteristics indicate that it could have a role to play in a superconducting compound.
"The idea eventually dawns on you, given that lithium when squeezed up is so good -- could we somehow combine it with beryllium and then get each to share the favorable characteristics of the other?" he said.
"We don't know yet whether it's going to be a fine superconductor," he added. But despite the high pressures involved (we live under about 1 atmosphere of pressure) -- modern technology makes creating the compounds and testing them relatively simple.
Still, a superconductor on Earth isn't much use if it requires constant squeezing to millions of atmospheres. And less simple than the squeezing is predicting whether the alloys will retain their peculiar structures when returned to normal atmospheric pressures.
So researchers are looking for new ways of doing the squeezing. That may mean looking beyond mechanical techniques to chemical elements or compounds that, added to the mix, could serve a compressing function.
"Some of the modern class of high-temperature superconductors have upwards of six elements in them," said Ashcroft. "Who's to say that some of the elements are providing the superconductivity side of things, and the others are actually providing the important bonding glue that makes it all go on under reasonable pressure?"
On the other hand, pressures of millions of atmospheres and higher are more common than one might expect.
"If you think about it, the 1 atmosphere that we live in is just a blithe accident of location," said Ashcroft. "It's superbly matched to biology, but most of the matter in the universe is under conditions of extreme pressure. There's a lot of territory out there."
By using two competing methods for predicting the molecular structures, Hoffmann noted, the interdisciplinary team proved the validity of each.
"Supposing you want to predict the structure of something that hasn't existed before," he said. One option is to look at related compounds and use those structures as models; the other is to program a computer to calculate the lowest energy structures given certain specifications.
"We used both methods," he said. "And I think we used both creatively."
He added: "This is just the beginning of a long trail of possibilities. There is so much room to play, even among the light elements."
Source: By Lauren Gold, Cornell University